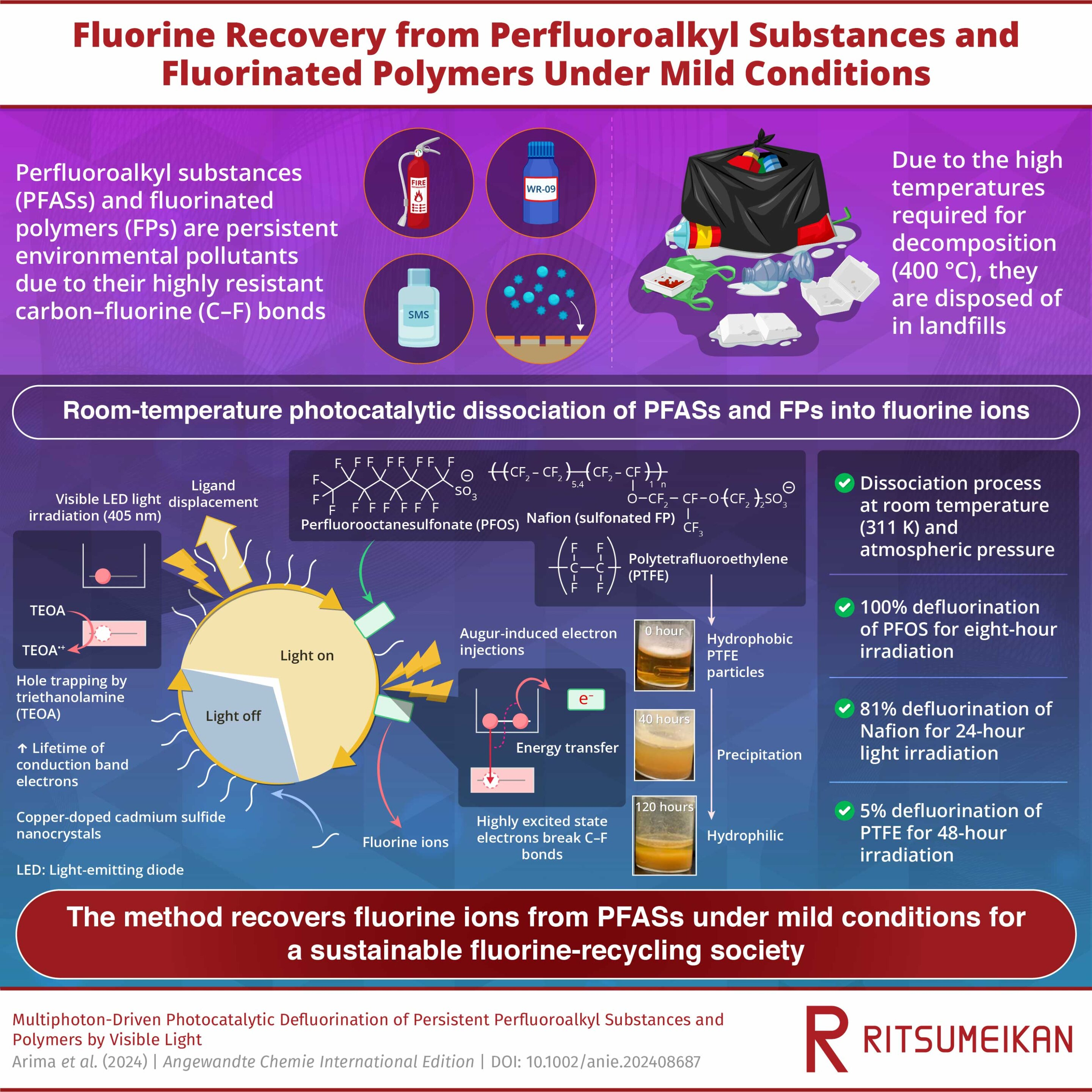
A room-temperature defluorination method proposed by researchers at Ritsumeikan University could revolutionize PFAS treatment
Phys.org Media Bias Fact Check Credibility: [High] (Click to view Full Report)
Phys.org is rated with High Creditability by Media Bias Fact Check.
Bias: Pro-Science
Factual Reporting: High
Country: United Kingdom
Full Report: https://mediabiasfactcheck.com/phys-org/Check the bias and credibility of this article on Ground.News:
- https://ground.news/find?url=https%3A%2F%2Fphys.org%2Fnews%2F2024-07-room-temperature-defluorination-method-chemicals.html
Media Bias Fact Check is a fact-checking website that rates the bias and credibility of news sources. They are known for their comprehensive and detailed reports.
Thanks to Media Bias Fact Check for their access to the API.
Please consider supporting them by donating.Beep boop. This action was performed automatically. If you dont like me then please block me.💔
If you have any questions or comments about me, you can make a post to LW Support lemmy community.Bias: Pro-Science
???
How could a science reporting website avoid a pro-science bias? For that matter what is a pro-science bias? Is it listening to experts in their fields or demanding studies for proof or something?
If you click into the link it defines “pro science bias”.
… using cadmium (also toxic) :
“The proposed method involves irradiating visible LED light onto cadmium sulfide (CdS) nanocrystals and copper-doped CdS (Cu-CdS) nanocrystals with surface ligands of mercaptopropionic acid (MPA) in a solution containing PFAS, FPs, and triethanolamine (TEOA).”I think elemental cadmium is toxic, I’m not sure CdS is. It is used in many many light detecting circuits. I think the CdS is used as a catalyst here anyway, so not depleted in use.
CdS is insoluble in pure water, yet :
Cadmium sulfide is toxic, especially dangerous when inhaled as dust, and cadmium compounds in general are classified as carcinogenic. (…) https://en.m.wikipedia.org/wiki/Cadmium_sulfide
ideally you will hope to have a process in which this powder will be supported on a stable substrate … but eventually, if you don’t use pure water, any impurity can have any chemical composition … so, if you are working in the real world with a mix of unknown soup, you will end up with chemical reactions … especially if there is some acids you will leach out the cadmium as soluble compounds or you may get fragmentation and powder coming out, going to the environment, pickied up by wind, creating dust in the air (…) .
Still, the process might be viable if well controlled and if the gain by eliminating PFAS is great enough.
P.S. this process involves liberation of fluoride so you will get :
Cadmium fluoride : Solubility in water 4.35 g/100 ml https://en.m.wikipedia.org/wiki/Cadmium_fluoride